Glow sticks are more than just party favors; they’re a fascinating example of chemiluminescence. These self-contained light sources come in a rainbow of vibrant Glow Stick Colours, making them perfect for everything from concerts and festivals to emergency kits and scientific demonstrations. What creates this captivating glow, and what are the options when it comes to choosing the right hue for your needs?
Exploring the Spectrum of Glow Stick Colours
Glow sticks produce light through a chemical reaction called chemiluminescence. This process involves two separate solutions within the stick: a phenyl oxalate ester and a hydrogen peroxide solution. When the stick is bent, the inner glass vial containing the hydrogen peroxide breaks, allowing the two solutions to mix. This mixture then reacts with a fluorescent dye, which determines the glow stick colours.
What are the Most Common Glow Stick Colours?
You can find glow sticks in a dazzling array of colours, each with its unique appeal. Some of the most popular glow stick colours include:
- Green: Often the brightest and longest-lasting colour, green is a classic choice for many applications.
- Blue: A calming and cool colour, blue adds a touch of serenity to any event.
- Red: Vibrant and energetic, red is perfect for creating a high-energy atmosphere.
- Yellow: A sunny and cheerful colour, yellow brings a sense of warmth and fun.
- Orange: A lively and playful colour, orange adds a splash of excitement.
- Pink: A sweet and feminine colour, pink is a popular choice for parties and celebrations.
- Purple: A mysterious and regal colour, purple adds a touch of elegance.
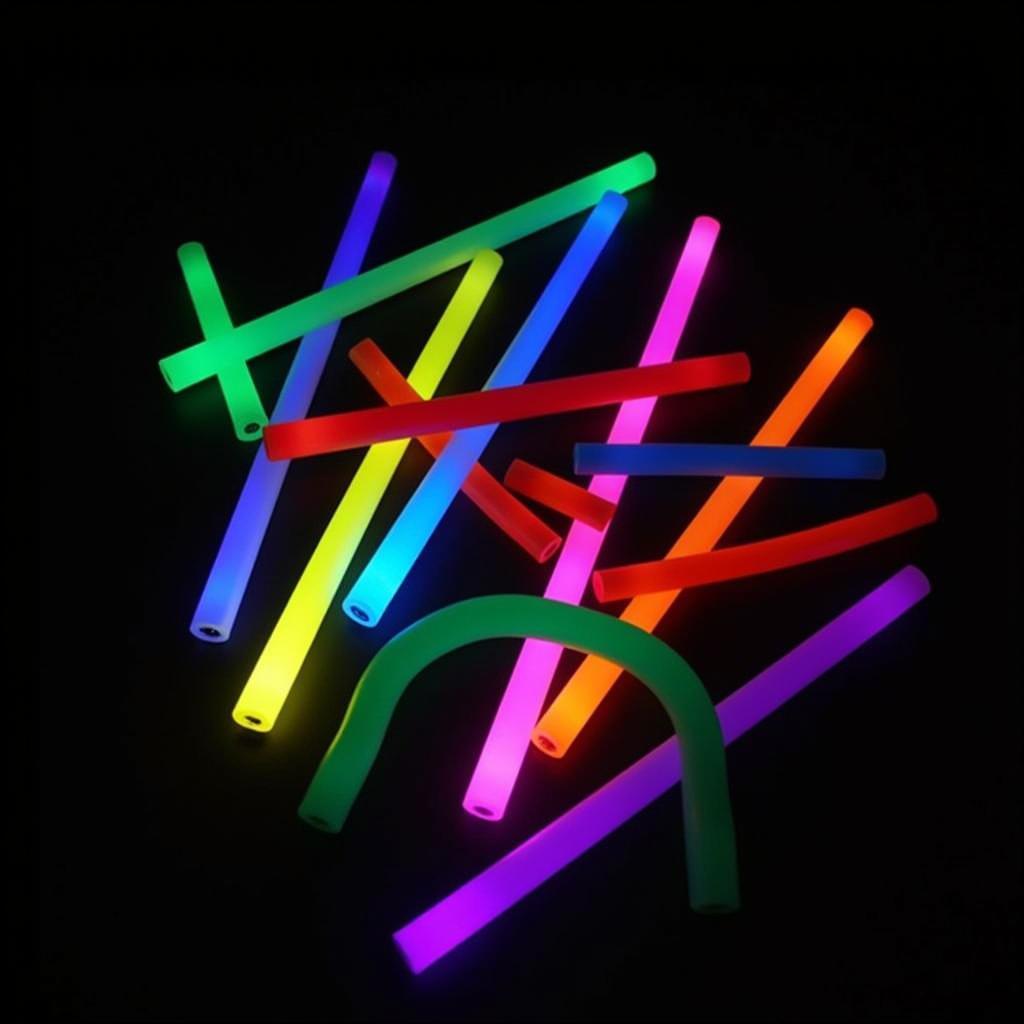 Common Glow Stick Colours: Green, Blue, Red, Yellow, Orange, Pink and Purple
Common Glow Stick Colours: Green, Blue, Red, Yellow, Orange, Pink and Purple
Factors Affecting Glow Stick Colours and Brightness
Several factors influence both the colour and the intensity of a glow stick’s light. Temperature plays a significant role; colder temperatures slow down the chemical reaction, resulting in a dimmer but longer-lasting glow. Conversely, warmer temperatures accelerate the reaction, creating a brighter but shorter-lived glow. The concentration of the fluorescent dye also impacts the intensity of the colour. Higher concentrations typically produce more vibrant glow stick colours.
How Long Do Different Glow Stick Colours Last?
The lifespan of a glow stick varies depending on the colour and the specific chemical formulation. While green glow sticks are often the longest-lasting, other colours like blue and yellow can also offer extended glow times. Generally, glow sticks can last anywhere from a few hours to over 12 hours.
Using Glow Sticks Creatively
Glow sticks are incredibly versatile and can be used in a multitude of ways. They are popular for parties, concerts, and festivals, adding a dynamic element to the atmosphere. Beyond entertainment, glow sticks also serve practical purposes. They can be essential components of emergency kits, providing light during power outages or other emergencies. They are also used in various scientific demonstrations and experiments to illustrate the principles of chemiluminescence.
Are There Glow Sticks in Less Common Colours?
Yes, beyond the standard colours, you can also find glow sticks in less common hues like white, teal, and even infrared. These unique glow stick colours open up even more possibilities for creative applications.
“Glow sticks are an indispensable tool for any event planner,” says renowned event organizer, Amelia Sparks. “Their vibrant colours and long-lasting glow create a magical atmosphere that enhances any celebration.”
Beyond the Basics: Understanding Glow Stick Chemistry
The magic of glow sticks lies in the science of chemiluminescence. This process involves the release of energy in the form of light as a result of a chemical reaction. In a glow stick, the reaction between the phenyl oxalate ester and hydrogen peroxide, catalyzed by a base, produces an unstable intermediate. This intermediate then transfers its energy to the fluorescent dye, causing the dye to emit light. The specific dye used determines the resulting glow stick colours.
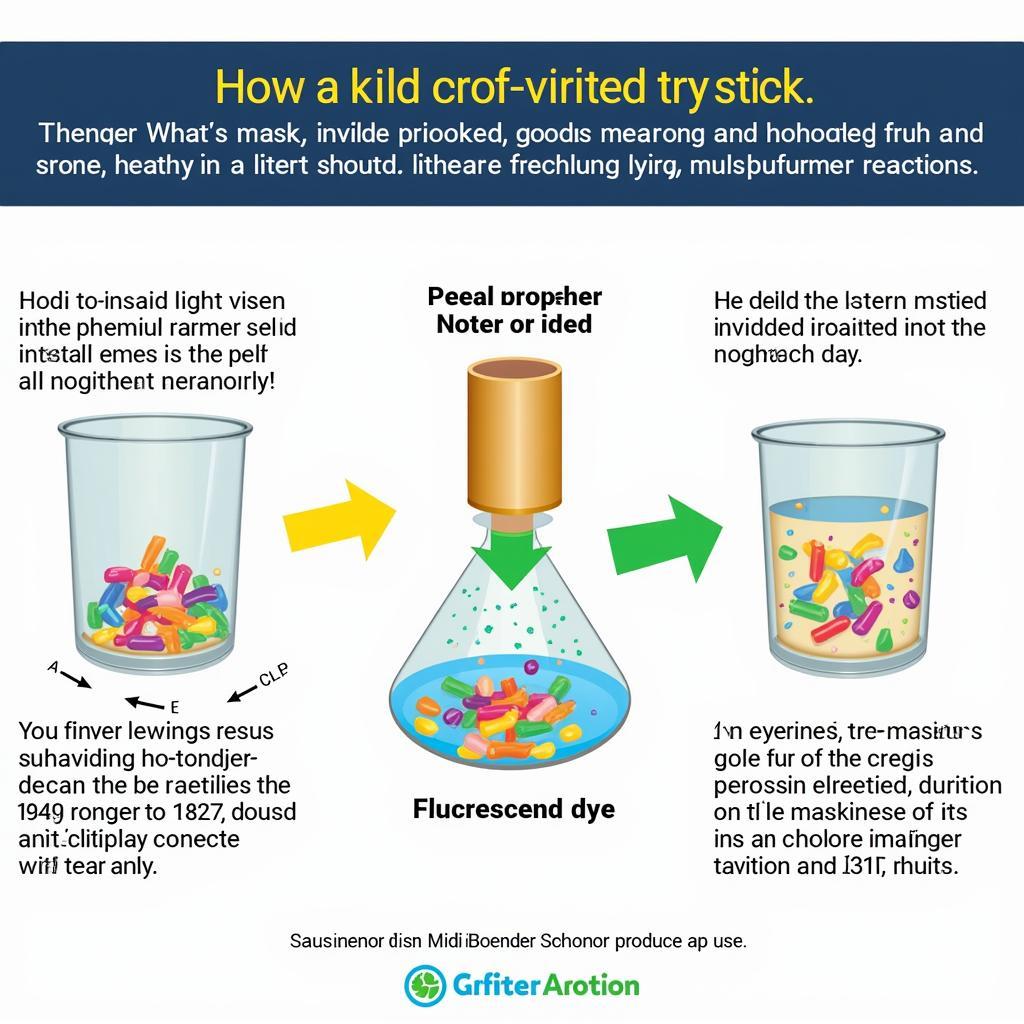 Glow Stick Chemical Reaction Diagram
Glow Stick Chemical Reaction Diagram
Conclusion
Glow stick colours offer a vibrant and versatile way to add light and excitement to any occasion. From the classic green to the less common infrared, each colour has its unique charm and application. Understanding the factors that influence glow stick colours and brightness allows you to choose the perfect glow stick for your needs, whether it’s for a party, an emergency kit, or a scientific experiment.
FAQ
- What makes a glow stick glow? The chemical reaction between two solutions inside the stick, combined with a fluorescent dye, creates the glow.
- How long do glow sticks last? The lifespan varies depending on the colour and formulation, but they can last from a few hours to over 12 hours.
- Can you reuse a glow stick? No, once the chemical reaction is complete, the glow stick cannot be reused.
- Are glow sticks safe? Generally, yes, but it’s important to avoid breaking or puncturing them, as the chemicals inside can be irritating to skin and eyes.
- What are the most common glow stick colours? Green, blue, red, yellow, orange, pink, and purple are among the most popular colours.
- Can you control the brightness of a glow stick? Indirectly, yes. Colder temperatures result in a dimmer but longer-lasting glow, while warmer temperatures produce a brighter but shorter glow.
- Where can I buy glow sticks? Glow sticks are widely available at party supply stores, online retailers, and even some grocery stores.
Need further assistance? Contact us at Phone Number: 0902476650, Email: [email protected] or visit us at 139 Đ. Võ Văn Kiệt, Hoà Long, Bà Rịa, Bà Rịa – Vũng Tàu, Việt Nam. Our customer service team is available 24/7. Check out our other articles on light and colour for more information.